Without battery, the vehicle becomes useless real estate - only rare modern cars can be started with a push. The battery is a power source for both the starter and for many electronic devices that are responsible for the comfort or safety of the car. But, unfortunately, any battery has a certain expiration date, after which it comes into disrepair. As a rule, the rechargeable batteries changed to new, but in some cases it is possible to repair a power source, after which it will serve its owner for another period of time. How to restore the rechargeable battery yourself - read further in the article.
Content
- How batteries are arranged
- How batteries work
- Types of automotive batteries
- The best is the most common type of battery for the car
- Acid battery, device and work principle
- Serviced and maintenance-free batteries, what is the difference
- Akb faults
- Remedy sulphation (step instruction) by the charge-discharge
- Tools, fixtures, consumables
- Table of electrolyte density ratio on battery charge degree
- The method of eliminating sulfate using reversible currents, advantages and disadvantages
- Washing the battery with subsequent charging, pros and cons
- Chemical method (fastest) sulfate removal (step-by-step instruction)
- Tips Pros: how to extend the service life of the battery
- How to find high-quality equipment at a reasonable price and free shipping on Aliexpress
How batteries are arranged
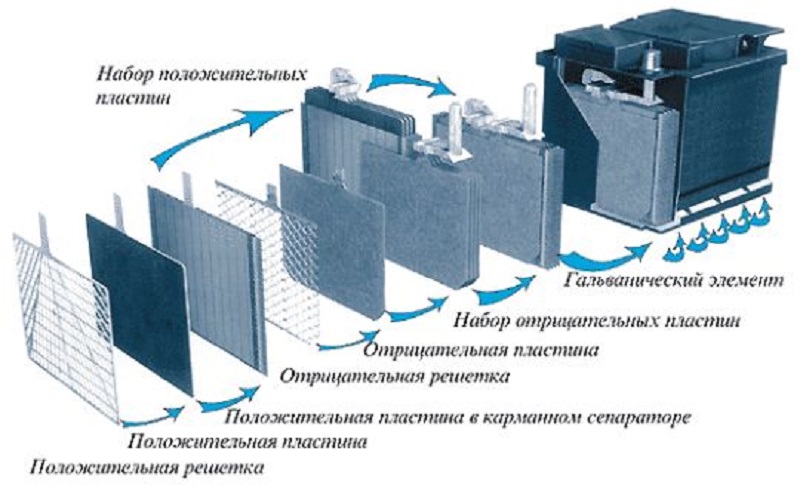
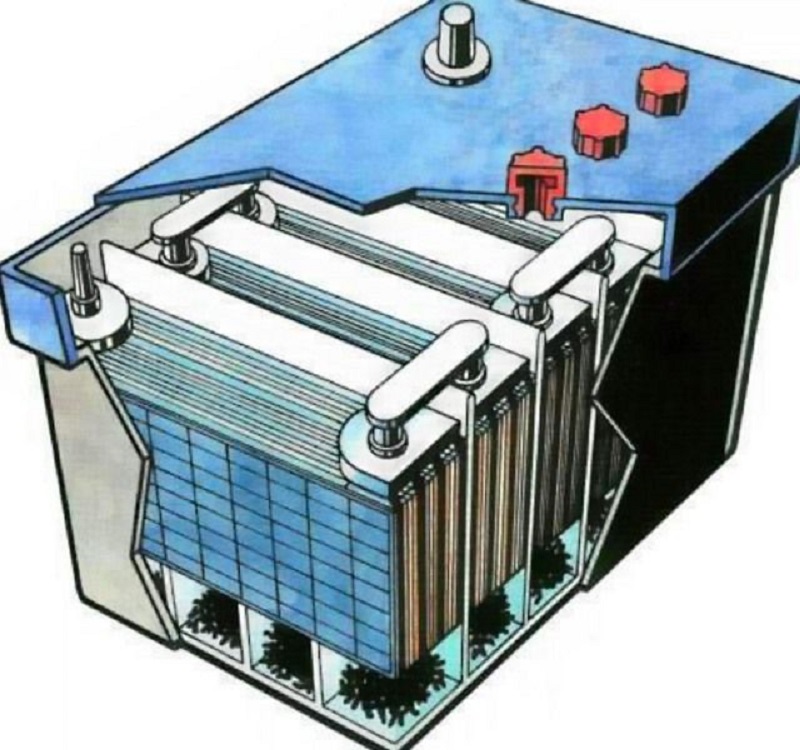
The rechargeable battery with a rated voltage of the twelve volt consists of (as a rule, six) autonomous batteries (that is, cans) of a smaller voltage (two volts), which are collected in one case and are consistently interconnected.

- The battery case is made from ebony or acid-resistant plastics. In the case there are compartments for mounting battery cans.
- The battery bank is a set of separable plates, isolated from each other with the help of acid-resistant separators.

- The pole plate has a lattice view and is made from lead. In the cells of this lattice, the active substance of a special composition and a porous structure in order to increase the area of \u200b\u200bcontact with electrolyte is also included. This substance is made from lead powder, which is added sulfuric acid. The negative plates are still added sulfate barium. In the process of forming the rechargeable battery, the plate is charged, and the active substance in the negative plates turns into sponge lead, in the positive - into lead dioxide.
- The electrolyte is designed to move the charged particles and is poured into the banks of the battery. It is made of distilled water and sulfuric acid.
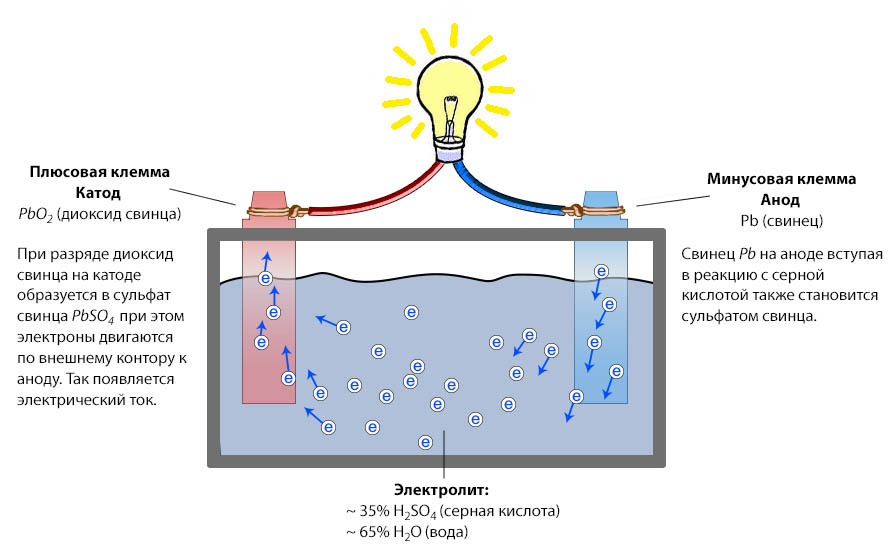
How batteries work
The principle of battery operation is very simple - when the load is connected, the charged particles in the battery begin to move, which entails the appearance of the current. When charging or the charger or the generator, the charge voltage exceeds the nominal value of the battery voltage and the particles are moving in the opposite direction.
Types of automotive batteries
To date, there are three types of car batteries - serviced, maintenance-free and partial maintenance.

Nowadays, the first type is rarely found. The housing of such batteries is performed from the ebony, and the outside is sealed, for example, mastic. In the served batteries, there is a possibility to replace any component.
Unnwided batteries do not require any human intervention during the entire service life. It uses a special design of the condensing system and plates. These batteries today are recognized as the highest quality, so their cost is very high.
The most common - partial service batteries. The essence of servicing such batteries is reduced only to maintaining the required level of electrolyte and controlling its density.
In addition, the batteries differ in technologies used in their production:
- Traditional batteries are lead acid. Here the electrolyte is a mixture of distilled water and rechargeable sulfuric acid.
- AGM technology is more modern. With this embodiment, the loss of the active substance on the plates is reduced to a minimum, which provides a longer resource and high power characteristics. When choosing a battery, it is worth considering that AGM batteries are recommended for use on vehicles equipped with energy recovery systems during braking, as well as numerous electricity consumers.

- As for gel batteries, the electrolyte is thiculated to a certain consistency with silica gel. Battery data, just like AGM batteries, provide greater current strength, regardless of the degree of discharge. Gel batteries are usually challenged.
The best is the most common type of battery for the car
The most common automotive batteries are acidic. Among the advantages of this type of batteries, their low cost, low self-discharge, as well as the absolute lack of "memory effect" should be noted.

Acid battery, device and work principle
Externally, acid battery looks like a closed plastic case, from which two terminals come. Inside the case is divided into six sections, where the working elements of the battery are placed - positive and negative lead plates on which the active mass is applied. They are arrangement. To eliminate the possible contact of these plates, there is a separator between them.
The plates are combined into blocks, each of which has an outlined jumper, that is, the barnet connected to the bridge. Thanks to Barteke, blocks of each bank are connected to one common bridge, having an output to the terminal.
The return of electricity in the battery is carried out as a result of chemical reactions, because the banks are filled with electrolyte. By itself, the battery does not produce electricity, it is, in fact, is simply a storage of electricity. When charging the battery, the electrical energy arriving on the terminals from the generator or the charger is converted to the chemical. During the discharge there is a reverse effect.
Served and maintenanceable batteries, what is the difference
The served batteries have small holes, closed by traffic jams located in the upper battery case. Domestic batteries with such holes are not equipped, they only have a small hole for gases. Their main difference is that serviced batteries require certain care from the owner, which is not quite convenient. Therefore, in our time they are used very rarely.

Akb faults
All battery malfunctions can be divided into internal and external. Independently detect them and eliminate every car owner, but it depends on the degree of damage
external how to eliminate
There are only two external faults - strong oxidation of the terminals, as a result of which the battery is poorly connected to the on-board network, and the breakfast of the body (or as a result of the external influence on it, or the crack on the case caused internal malfunctions).
As for the terminals, there is nothing to say here. Look, whether there are a significant layer of oxides. In the presence of this layer, it is written.
If there is a breakdown in the case, it is easy to detect it, the electrolyte will flow out of it. Crack, if any, can be seen, but when the battery is served. The electrolyte is drained from the battery, and then close the crack. To do this, you use a soldering iron and a piece of plastic. First, the crack itself is suspended, and then the prepared plastic is suspended over the prepared plastic for greater confidence as the work done. At the last stage, we check the tightness of the body, the bay of distilled water into it.
internal malfunctions
Internal malfunctions arising in the battery are significantly larger, and most of them makes a battery harm, eliminate which is impossible. One of the most common accumulators faults is plate sulfate.
aKB sulfate, reasons, is it possible to eliminate
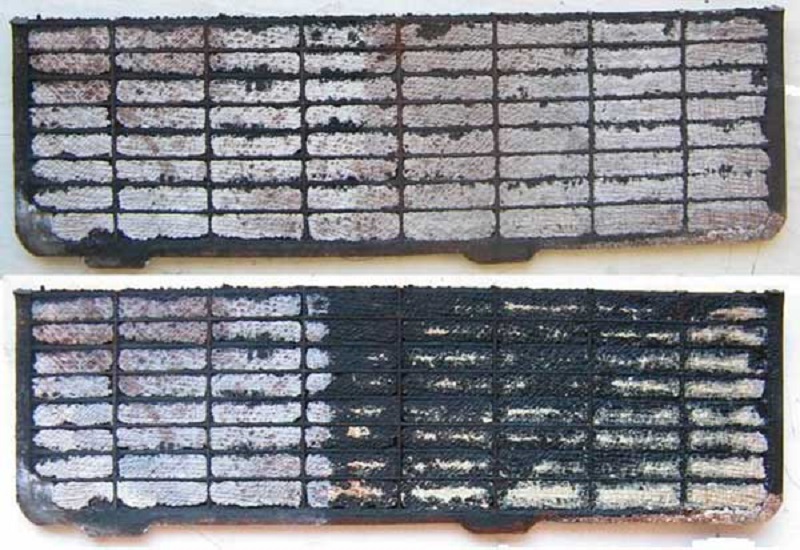
To sulfate the battery, it leads to its incorrect operation - long-term storage of the battery in the discharged state, constant short-term batteries, frequent deep discharges, so it is necessary to pick up the battery on the vehicle brand. In fact, sulfate is the appearance of a sulfate lead on the surface of the plates, which is why the electrolyte is not capable of penetrating inside the active mass, so a certain part of this mass is no longer able to react.
Resistance inside the battery increases, which entails a decrease in capacity. As a result, the battery cannot take the charge completely and quickly discharged. Sulfate plates in the early stages can be eliminated, however, if it is deep - the battery repair is not subject to repair.
swimming of battery plates, reasons how to eliminate
There are also breakdowns such as shrinking from the plates of active mass, with a possible further closure. When unscrewed, it usually helps washing batteries with distilled water. It is also possible to bloating the battery as a result of the freezing of the electrolyte. This happens if the discharged battery was on a strong frost. After freezing, the car battery is not subject to recovery.
Sulfate elimination methods (step-by-step instruction) by charge-discharge
Methods for eliminating plate sulfate are used by several. The first, most common way is to conduct a control cycle (abbreviated CTC). The use of this method will provide an opportunity to eliminate sulfate in the early stages, as well as restore the battery capacity.
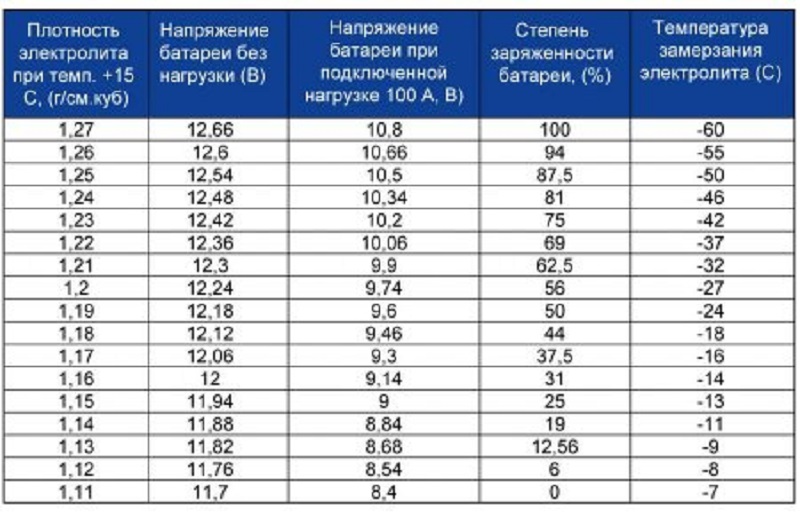
The essence of this method is to hold a charge-discharge cycle. First, the battery charging is performed. The charging of the battery is performed by a current that is valid for ten percent of the nominal capacity, i.e., with a battery capacity of sixty Ah, the current strength should be six amps. After charging, density checks in each bank.
In a fully charged battery, this indicator should be 1, 27. When this value is below, it will be necessary to bring the density to the desired value with further accumulation of the battery for half an hour for mixing the electrolyte.
After charging, a control discharge is performed, for which the source of energy consumption is connected to the battery terminals. The consumption of energy at the same time a connected consumer should not exceed ten percent of the tank. As a consumer, it is best to apply a car heating lamp having a certain power.
Calculate the required power by multiplying the voltage and current force. The strength of the current in the calculation process is taken on the basis of the battery tank. That is, in the process of calculating the power required for the discharge of the battery by sixty Ah, the current of the current is taken by six amps, the value is multiplied by 12 V. As a result, we obtain the power value of 72 W. Approximately such power and should be at the lamp.
Then the battery is discharged with the lamp, while systematically measured the voltage. Battery Battery It is necessary to achieve a reduction in voltage at the Accord terminals to 10.2. This voltage value will indicate the full battery discharge. At the same time it is necessary to measure the time for which the battery was discharged. A new battery has this value to be about ten o'clock. The smaller the discharge time, the more battery lost its container. It is impossible to leave the discharged battery for a long time, it is immediately necessary to put it for charging until the charge is completely restored.
When performing this event, the battery capacity will be restored, and as a result of a decrease in sulfation, the internal resistance will decrease.
Tools, fixtures, consumables
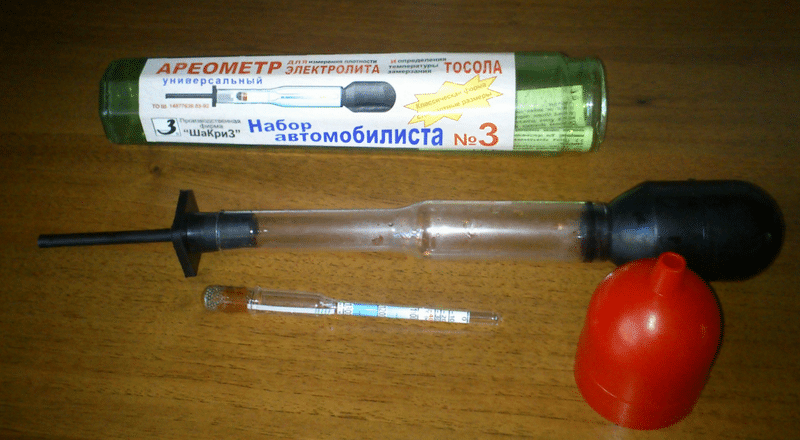
To conduct a control and training cycle, you will need the presence of a charger, a voltmeter, aireometer, as well as an electrical energy source.
Table of electrolyte density ratio on battery charge degree
The method of eliminating sulfate using reversible currents, advantages and disadvantages
The second method of removing sulfate is to use reversing currents while charging the battery. The disadvantage of this method is the need for special equipment - reversing current generator. The essence of this method is reduced to the long charge of the battery with small currents. Thus, with a non-essential sulfate, the battery is charged with a minor current - 0.5-2 A. Charging is made long period, and in some cases it can reach fifty hours.
The end of the desulfation process is non-changeability of voltage on terminals and an unchanged electrolyte density over two or more hours.
Washing the battery with subsequent charging, pros and cons
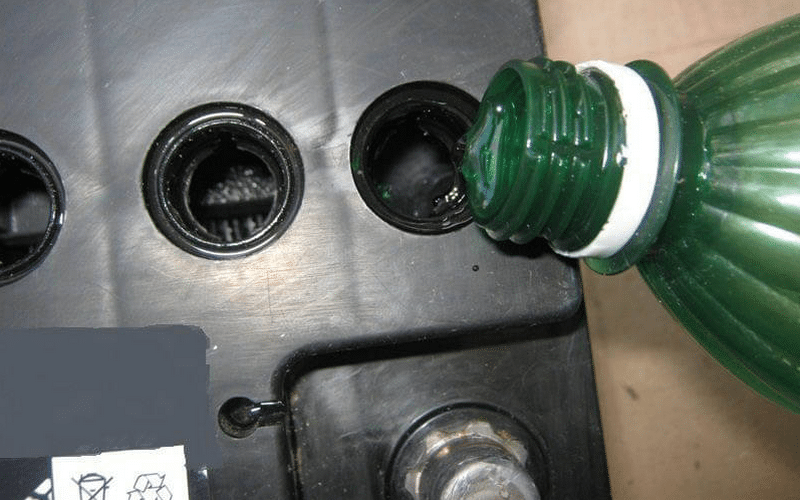
The third method used to restore the battery is washing the battery with subsequent charging. However, this method is long and its execution is able to delay as much as a month. The battery is drained with electrolyte, and distillate poured into its place. The battery is then charged in voltage conditions of 14 V.
After the distillate boils, the voltage is slightly reduced. The main task is to maintain boiling in the battery, but not intense. Distillete density over time will increase due to the dissolution of lead sulfate in water. Then the water is drained and poured a new one, and the battery is postponed with a slight voltage.
It must be achieved that in distillate bubbles arise, however, it is not necessary to bring it up. On charging, the battery should be kept until the density stops changing within a few days.
Chemical method (fastest) sulfate removal (step-by-step instruction)
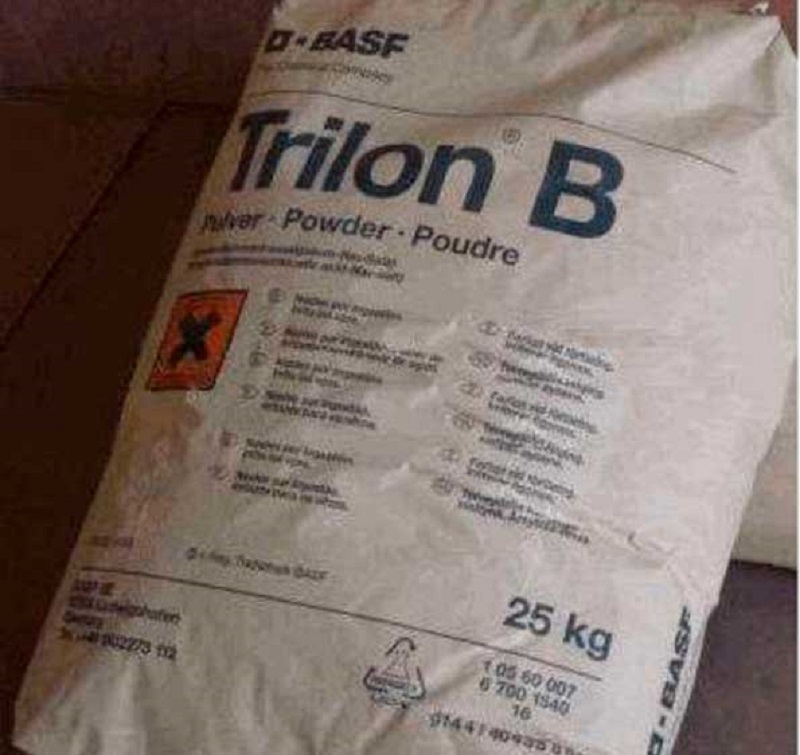
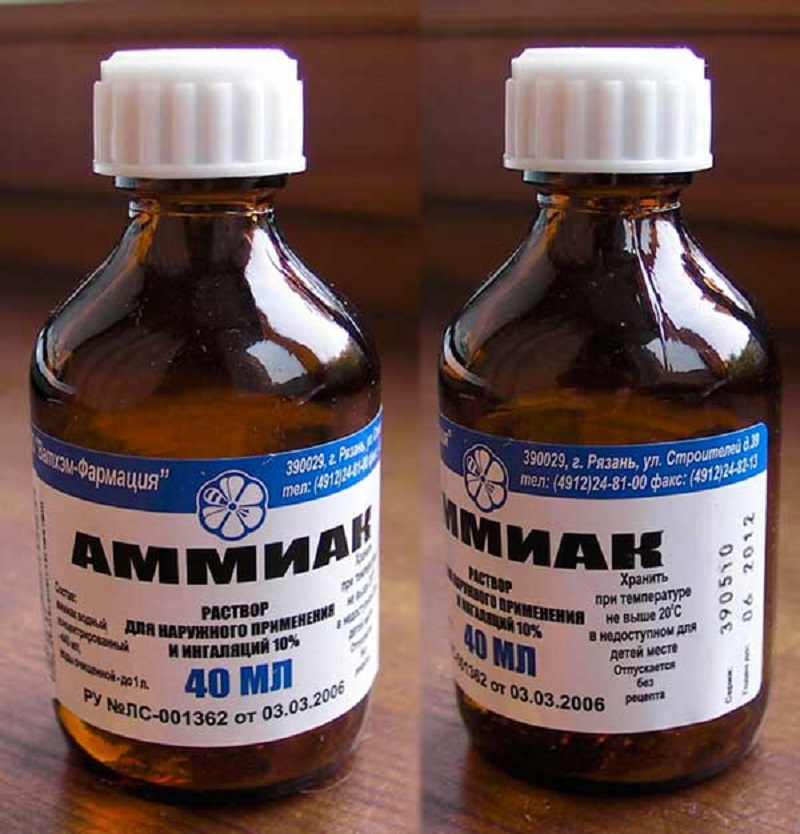
The fastest method of removing sulfation is chemical. It comes down to flushing the battery with a trildon b and ammonia. Before washing with a solution, the battery is charged, the electrolyte is drained and washed with distillate. Next, the jars are poured with an aqueous solution with the addition of five percent of the volume of ammonia water and two percent - Trilon B.
This and sulphate solutions react, which will be accompanied by splashes and boiling. As soon as boiling is over, the solution is drained, and the cans are washed with water, after which the electrolyte is poured and charged the battery.
Tips Pros: how to extend the service life of the battery
All malfunctions with the battery do not appear by themselves, they occur as a result of negligent exploitation and ignoring systematic services. The battery does not require a lot of attention. It is enough at least once in half a year to charge it using a charger.
If the battery is served, before recharging, you must pay attention to the level of electrolyte and need to restore it. After charging, check the electrolyte density in each bank. Significant differences in the density values \u200b\u200bbetween banks should not be. The minimum difference between them is allowed.
Before mounting a new battery on a car, check the voltage that the generator gives out, to eliminate the recharge. In addition, establishing a new battery, it is necessary to secure it well to prevent possible damage to the case.
How to find high-quality equipment at a reasonable price and free shipping on Aliexpress
- 1 step - registration on the site, for which you need to enter the name, name and email address, and also come up with a password. To your account on Aliexpressnot blocked, it is important to confirm email during the day.
- 2 step - fill the shipping address. This can be done in your profile. It is important to fill all the fields in Latin characters.
- 3 step - near the graph of the category click on the "Watch all" link (in the upper left corner of the site).
- 4 step - choose the category "Cars and Motorcycles".
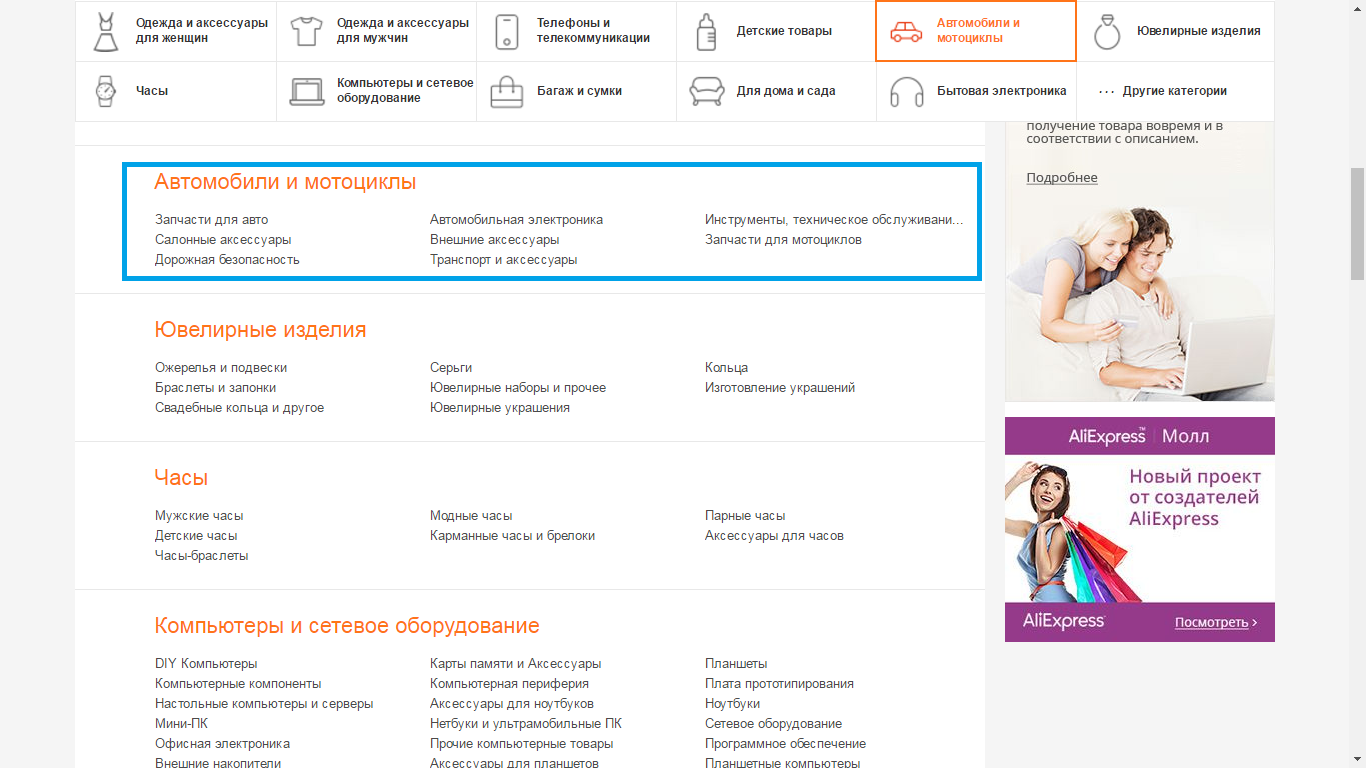
- 5 step - then you will see eight subcategories, namely: Spare parts for motorcycles; Spare parts for cars; Tools, maintenance; automotive electronics; transport and accessories; salon accessories; external accessories; Road safety. From these categories, choose the required, depending on the type of item. For example, salon accessories.
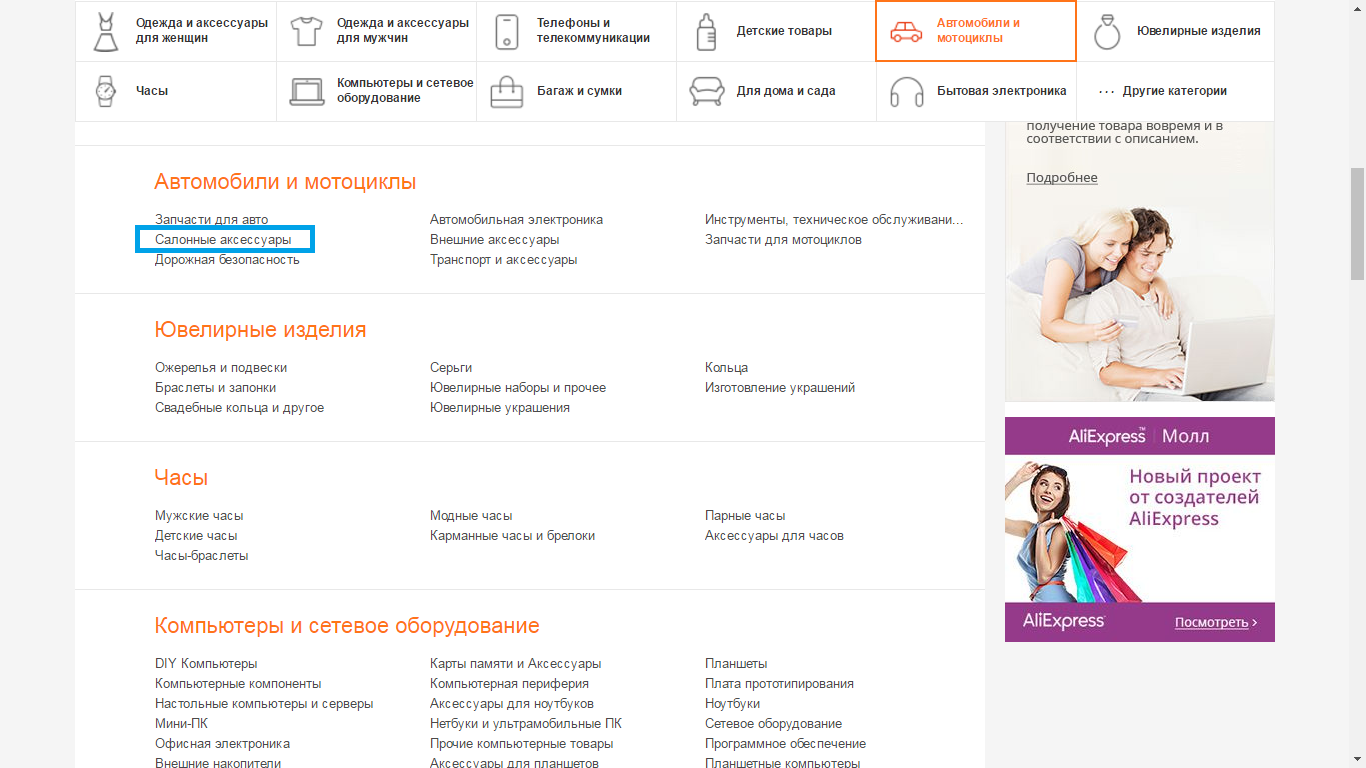
- 6 step - in the search string I enter keywords, for example, covers for automotive seats.
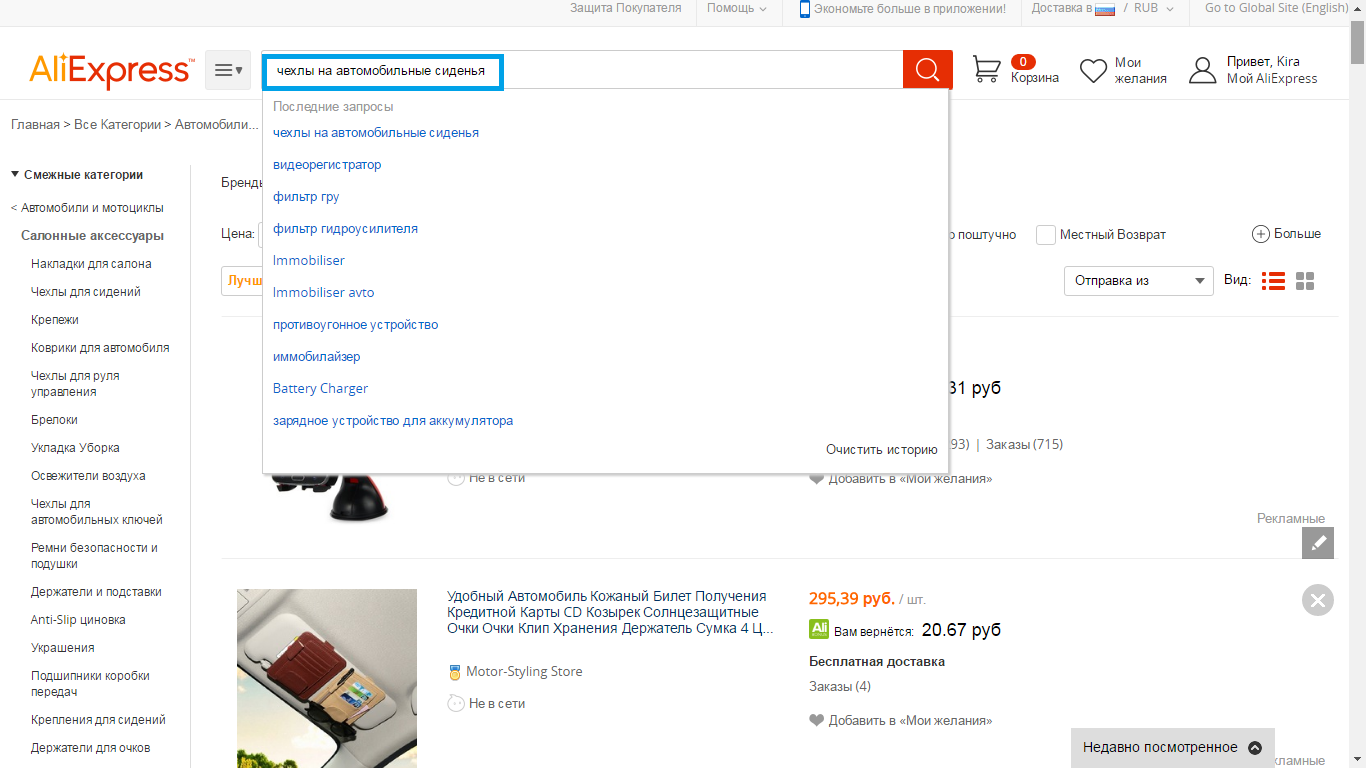
- 7 Step - At the top of the page, you will see the toolbar, with which you can sort the results and cut off unnecessary. For example, we choose only retail goods and goods with free shipping. As for sorting results, it is better to choose sorting by the seller rating. Why? Yes, because if the seller has a high rating, then its goods are high-quality, comply with the description and inexpensive. By the way, do not forget to read the reviews of other buyers.
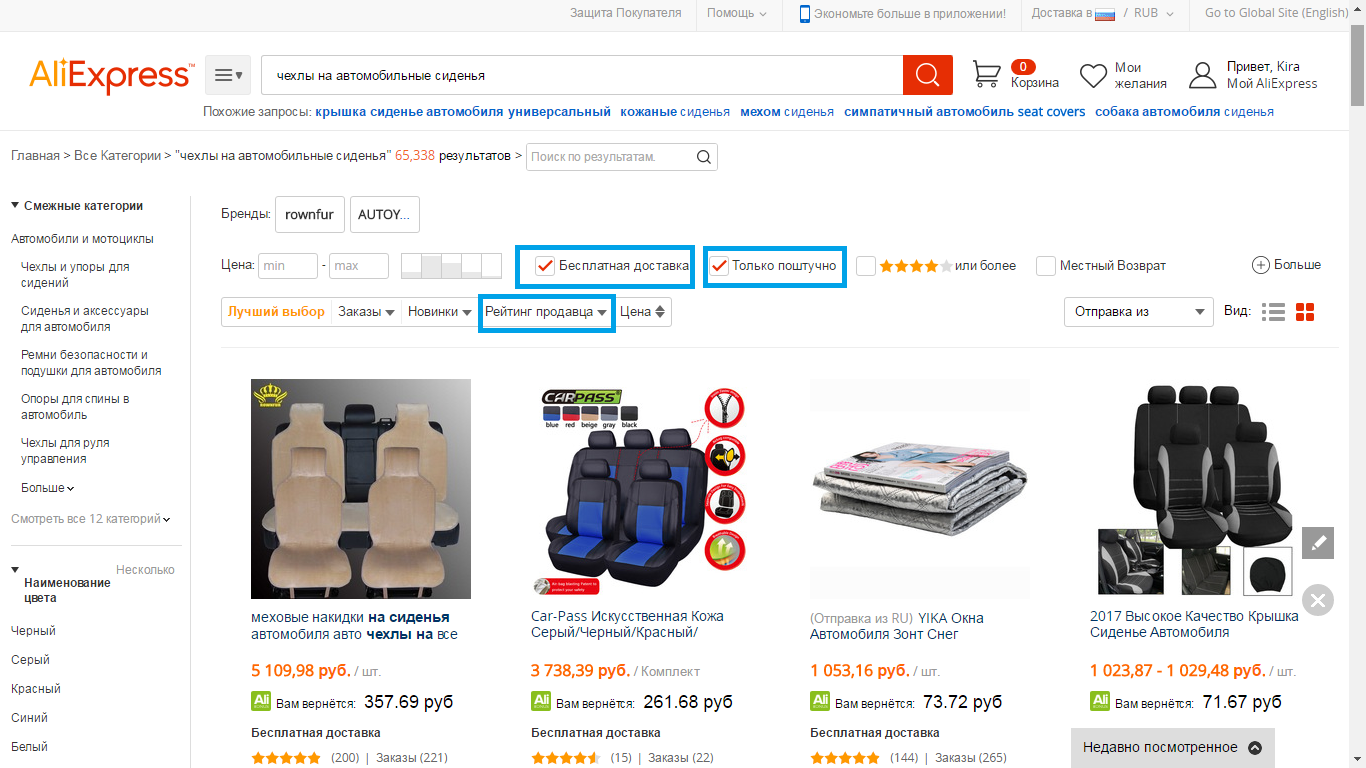
- 8 step - on the product description page, you must select the quantity you need, size and color.
- 9 Step - If you want to go to pay for goods right now, click on the "Buy Now" link, if you want to pay for the goods a little later, click "Add to Cart".
- 10 And the last step is the payment of goods.
Related Materials
- Stove 2110, bad warm stove 2110, VAZ 2110 heating system, repairing the heating system VAZ 2110 with their own hands
- VAZ 2114 stove blows with cold air, stove 2114, bad warm stove VAZ 2114, device and repair of heating VAZ 2114 do-it-yourself, removing the stove VAZ 2114
- How to subdominize the car. How to put a jack. Types of jacks for cars.
- VAZ 2109 Fuse Block, VAZ 2109 Fuse Block Carburetor, VAZ 2109 Fuse Block Injector, Old VAZ 2109 Fuse Block, VAZ 2109 Fuse Block, VAZ Fuse Block 2109
- Car exhaust gas catalyst, faulty catalyst, pluses and cons of the catalyst, how to change the catalyst for the planeencitel
- Stove blowing cold air VAZ 2114, badly blowing the stove VAZ 2114, why badly blowing the stove VAZ 2114
- How to find out the owner of the car by the number of his car, check the car by the number of the traffic police machine, check the car by the state number of the car for free
- How to choose Used tires, Useful Tips
- Winter car road, pressure in passenger car tires in winter, good battery for the car in winter, whether to warm the car in winter
- In winter, the car is poorly started. How to make a car in winter, do you need to warm up the car in winter, useful tips
- Economy fuel consumption machines, the most economical car consumption
- Tires brands for passenger cars, labeling of car tire labeling, residual passenger car tire protector, how to pick a tire on a car brand, car tire tread pattern
- Working transmission operation, mechanical gearbox clutch work, driving with manual gearbox, useful tips
- Rear beam Peugeot 206 sedan, rear beam device Peugeot 206. Rear beam Peugeot 206 Malfunction, repair of the rear beam Peugeot 206
- Diesel fuel in winter, additive for diesel fuel in winter, how to choose the best diesel fuel
- Diesel winter does not start. How to start diesel in winter, heating diesel in winter.
- Japanese bridgestone tires, winter studded bridgestone tires, bridgestone tires brand
- Tire marking decoding for passenger cars, labeling wheels, how to choose the right tires on the disks
- Diesel engine in winter, launch of the diesel engine in winter, what oil to fill in a diesel engine in winter, useful tips
- LED backlight of the car, the backlight of the bottom of the car, the backlight of the legs in the car, the backlight in the door of the car, the backlight of the car is fine
- Recovered tires, bus tire, restored tire protector, can I use them
- Choose winter tires, which is a winter tires, which pressure in winter tires should be marked with winter tires, how to choose the right winter tires, the best winter tires 2019
- Steering rail rail, knock of steering rack, reasons for the knock and repair of the steering rack do it yourself
- Cameless car tires, a set for repair of tubeless tires, repair of the cannon-free tire do it yourself
- Russian tires, Russian tires Winter, Russian All-season tires, Voronezh AMTEL tires, Tires "Matador Omsk Tire", Kama-tires are world-class bus
- How to open a car without a key. Lost the key from the car what to do, the key from the car inside the car
- Silent tires, quiet winter tires, quiet studded bus, which tires to choose, overview tires
- Tires and safety, safety of the bus, why it is necessary to constantly monitor car tires
- Rules of safe driving of the car in the rain and slush, safe driving of the car for beginners
- Rust converter which is better for cars, rust converters to choose how to use rust transducer, professionals
- Polishing the body of the car do it yourself, how to choose a polishing paste, useful tips
- Engine durability, engine life, how to extend engine life
- Knock in the car. Knock when moving a car. What can knock in the car. How to determine the cause of the knock.
- ABS car, what is ABS car, ABS system malfunction, ABS diagnostics
- Overtaking a car when you can start overtaking a car, rules of traffic rules
- Fuel pump VAZ 2110, VAZ 2110 gas station scheme, VAZ 2110 fuel pump device, VAZ 2110 gas station repair,
- Automotive antennas for radio, automotive antenna device, car antenna do it yourself
- Front suspension Kalina, device front suspension Kalina, knock in front suspension Kalina, repair of front suspension Kalina
- Shock absorber Oil, best oil shock absorbers, pumping oil shock absorbers, how to properly pump oil shock absorber
- Clutch malfunctions, touches clutch, causes a clutch malfunction, how to eliminate





























If the recovery and charging the battery charge is still relevant, I propose a scheme of a simple device for recovering batteries and individual batteries. Performance: For example, with the help of such a scheme, an absolutely dead car battery was restored overnight, which cannot charge the standard charger. Recovery time - 10 hours, current - 0.15 A. After this procedure, the car started the car in the morning, but the battery was still recharged to the full capacity of the charger (industrial manufacture). The same homemade device has been extended by a multitude of batteries from phones and tablets. Details: Transformer with secondary winding current up to 1 A and output voltage up to 15-20 V, variable resistor - wire [used PPB-15G] resistance 0.3 - 1.5 com, diode - any rectifier 1-1.5 A, a condenser 10 ... 22 μF to 16 ... 50 V. Since this site is impossible to attach a photo of the scheme on this site, so I will try to explain in words. One output of the secondary winding of the transformer will be minus and joins the minus of the recoverable (charged) battery. Parallel to the secondary winding of the transformer, a variable wire resistor with a capacity of at least 15 W is soldered, the engine of which is soldered to the dioda anode and minus the condenser. The cathode of the diode and plus the capacitor solder together - it will be a plus output. To indicate charging and protect against accidental short circuit between the output of the plus and minus, the lamp (12 V, 1-2 A) is not bad in the break of this advantage wire. Also to control the current in series with a lamp, you can turn on the armeter (for current to 1 A, with polarity compliance). The only disadvantage of the device is no protection against recharging the battery, therefore it requires constant charging current control and charging time.