Electrolytes are solutions capable of carrying out electric current. These include acids, salts and bases, that is, substances having strongly polar and ionic ties. The batteries in the electrolyte occurs a chemical reaction that allows you to accumulate electrical energy and give it. The quality of the electrolyte in the battery determines how many charging cycles it will endure.
Content
- Electrolyte for battery with your own hands, can I make
- Electrolyte density
- Sulfuric acid density
- Preparation of the necessary tools, materials and reagents for the manufacture of electrolyte with their own hands
- Safety and Protective Special Security
- Work on the preparation of electrolyte with their own hands in stages
Electrolyte for battery with your own hands, can I make
The electrolyte for batteries can be, both to buy in specialized stores, and make it with their own hands, having sufficient knowledge.

Before proceeding with the manufacture of electrolyte, you need to carefully examine the instructions for the manufacture and the list of what will be needed for this, and will also learn how to measure its density correctly.
Electrolyte density
The electrolyte density parameter has a very large impact on the operation of the battery, you can determine the status of the battery.
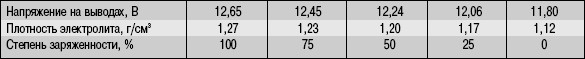
It must be periodically verified. Elevated density causes corrosion in the battery. With a reduced density in a minus temperature, the electrolyte in the battery can simply freeze. Also a decrease in the density value means that there has occurred, breaking the chain or in some of the cells there is a defect. A deep discharge of the battery may occur, while the density decrease will occur in all cells.
Sulfuric acid density
The electrolyte of most batteries today consists of a solution of concentrated sulfuric acid and distilled water. Measure electrolyte density will not be much difficulty, but there are some nuances.
Density measurement can be performed only in the batteries serviced. In which access to banks is present, that is, there are fuel holes (usually six of them). Through these holes, electrolyte is made to measure the density.
Density should be measured when the battery is charged. The temperature should be about 20 degrees. To measure its density, you will need a special device called an areaometer.
This device is used to measure the density of the liquid, it can be purchased in any of the automata.
We will proceed to density measurements:
- Unscrew the plug holes;
- Immerse the aerometer tip to the electrolyte through the hole;
- Press the pear and slowly type the electrolyte. The float in the aerometer should swim freely, but do not rest in this pear, otherwise it will show not the correct testimony.
Density value We look at the float, it must be 1250-1270 kg / cm3 or 1.25-1.27 g / cm3, respectively.
This method needs to check the density in all banks of the battery, that is, take the sample from each bay hole. If the density does not correspond to this value, then it must be increased or lower.
Increase and decrease the electrolyte density in the battery
To change the density, it is necessary to pump a small amount of electrolyte from the battery bank, and add distilled water or electrolyte with an increased density to increase it.
Repeat this process is necessary before the density does not meet the recommended. Adjusting the electrolyte density takes a large amount of time. After each procedure, you must put the battery for charging for half an hour, after charging, wait from an hour to two and re-measure the density. We also do not forget about the level of electrolyte in the battery.
Preparation of the necessary tools, materials and reagents for the manufacture of electrolyte with their own hands
Prepare the electrolyte for the battery is easy, for the preparation of electrolyte we will need:

- Distilled water (in its absence, it is possible to use rainwater, the use of water assembled into iron capacity is not allowed;

- Special accumulator sulfuric acid;
- Resistant to acid capacity, a volume of at least 5 liters (not allowed by the use of glassware, it can burst from heating);
- Acid resistant wand;
- 5% soda solution.
All the dishes should be clean.
Attention! Sulfuric acid and distilled water should be clean! Impurities even in small quantities will reduce battery life.
Safety and Protective Special Security
During the preparation of electrolyte, be extremely attentive and especially careful, since it includes pure sulfuric acid.

If you get into open areas of the skin, it will leave strong chemical burns. During the preparation of electrolyte, be sure to use special protective glasses, rubberized gloves, special clothes (bathrobe or rubberized apron) and rubber shoes.
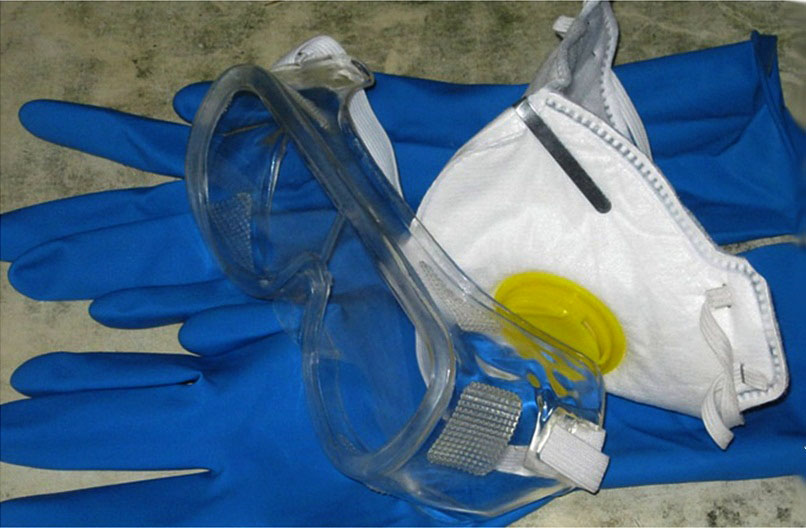
When sulfuric acid gets into the skin, rinse this section with distilled water and treat it with soda solution.

Be sure to use the glasses as it is an almost 100% probability of loss of vision.
Work on the preparation of electrolyte with their own hands in stages
When you have everything you need at hand, and clothing is dressed on you, you can begin the preparation of electrolyte:
- Initially, a stable container pour clean distilled water.
- After that, it is extremely careful that we carefully tighten with sulfuric acid, while neatly stir our solution, an acid-resistant stick. Acid to add small portions, thin jet. When the acid is added to the water, it is immersed in the thickness of the water as a result of which its splashing does not occur. Pay special attention that when water mixed with acid can stand out a huge amount of heat due to which glassware can burst, and plastic melt. Approximate ratio is 310 grams of sulfuric acid per liter of water.
- After each teller, check the density of the solution.
Attention! Do not pour water into acid! A stormy chemical reaction may occur, accompanied by splashing sulfuric acid from the tank.
As you see in the preparation of electrolytes with your own hands there are no any difficulties. The main thing is to show special care and comply with all security requirements.
Related Materials
- Stove 2110, bad warm stove 2110, VAZ 2110 heating system, repairing the heating system VAZ 2110 with their own hands
- VAZ 2114 stove blows with cold air, stove 2114, bad warm stove VAZ 2114, device and repair of heating VAZ 2114 do-it-yourself, removing the stove VAZ 2114
- How to subdominize the car. How to put a jack. Types of jacks for cars.
- VAZ 2109 Fuse Block, VAZ 2109 Fuse Block Carburetor, VAZ 2109 Fuse Block Injector, Old VAZ 2109 Fuse Block, VAZ 2109 Fuse Block, VAZ Fuse Block 2109
- Car exhaust gas catalyst, faulty catalyst, pluses and cons of the catalyst, how to change the catalyst on the planeencitel
- Stove blowing cold air VAZ 2114, badly blowing the stove VAZ 2114, why badly blowing the stove VAZ 2114
- How to find out the owner of the car by the number of his car, check the car by the number of the traffic police machine, check the car by the state number of the car for free
- How to choose Used tires, Useful Tips
- Winter car road, pressure in passenger car tires in winter, good battery for the car in winter, whether to warm the car in winter
- In winter, the car is poorly started. How to make a car in winter, do you need to warm up the car in winter, useful tips
- Economy fuel consumption machines, the most economical car consumption
- Tires brands for passenger cars, labeling of car tire labeling, residual passenger car tire protector, how to pick a tire on a car brand, car tire tread pattern
- Working transmission operation, mechanical gearbox clutch work, driving with manual gearbox, useful tips
- Rear beam Peugeot 206 sedan, rear beam device Peugeot 206. Rear beam Peugeot 206 Malfunction, repair of the rear beam Peugeot 206
- Diesel fuel in winter, additive for diesel fuel in winter, how to choose the best diesel fuel
- Diesel winter does not start. How to start diesel in winter, heating diesel in winter.
- Japanese bridgestone tires, winter studded bridgestone tires, bridgestone tires brand
- Tire marking decoding for passenger cars, labeling wheels, how to choose the right tires on the disks
- Diesel engine in winter, launch of the diesel engine in winter, what oil to fill in a diesel engine in winter, useful tips
- LED backlight of the car, the backlight of the bottom of the car, the backlight of the legs in the car, the backlight in the door of the car, the backlight of the car is fine
- Recovered tires, bus tire, restored tire protector, can I use them
- Choose winter tires, which is a winter tires, which pressure in winter tires should be marked with winter tires, how to choose the right winter tires, the best winter tires 2019
- Steering rail rail, knock of steering rack, reasons for the knock and repair of the steering rack do it yourself
- Cameless car tires, a set for repair of tubeless tires, repair of the cannon-free tire do it yourself
- Russian tires, Russian tires Winter, Russian All-season tires, Voronezh AMTEL tires, Tires "Matador Omsk Tire", Kama-tires are world-class bus
- How to open a car without a key. Lost the key from the car what to do, the key from the car inside the car
- Silent tires, quiet winter tires, quiet studded bus, which tires to choose, overview tires
- Tires and safety, safety of the bus, why it is necessary to constantly monitor car tires
- Rules of safe driving of the car in the rain and slush, safe driving of the car for beginners
- Rust converter which is better for cars, rust converters to choose how to use rust transducer, professionals
- Polishing the body of the car do it yourself, how to choose a polishing paste, useful tips
- Engine durability, engine life, how to extend engine life
- Knock in the car. Knock when moving the car. What can knock in the car. How to determine the cause of the knock.
- ABS car, what is ABS car, ABS system malfunction, ABS diagnostics
- Overtaking a car when you can start overtaking a car, rules of traffic rules
- Fuel pump VAZ 2110, VAZ 2110 gas station scheme, VAZ 2110 fuel pump device, VAZ 2110 gas station repair,
- Automotive antennas for radio, automotive antenna device, car antenna do it yourself
- Front suspension Kalina, device front suspension Kalina, knock in front suspension Kalina, repair of front suspension Kalina
- Shock absorber Oil, best oil shock absorbers, pumping oil shock absorbers, how to properly pump oil shock absorber
- Clutch malfunctions, touches clutch, causes a clutch malfunction, how to eliminate











Comments